Abstract
Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that serve as essential mediators of humoral immunity. These highly specific proteins recognize and bind to foreign antigens, initiating a cascade of immune responses that protect the host from pathogens and toxins. Immunoglobulins are characterized by their remarkable diversity, with five main classes (IgG, IgM, IgA, IgD, and IgE) each possessing distinct structural features and biological functions. This article comprehensively examines the structure, classification, and functions of immunoglobulins, with special emphasis on the unique properties of immunoglobulins found in camel milk, which contain heavy-chain-only antibodies with significant therapeutic potential.
1 Introduction to Immunoglobulins
Immunoglobulins, commonly known as antibodies, are glycoprotein molecules produced by plasma cells (differentiated B lymphocytes) in response to specific immunogens. These molecules constitute approximately 20% of total plasma proteins and play indispensable roles in humoral immunity against pathogens, toxins, and other foreign substances. Initially discovered in 1890 by Emil von Behring and Kitasato Shibasaburo as “neutralizing substances” against Corynebacterium diphtheriae, immunoglobulins have since been recognized as essential components of adaptive immunity with remarkable specificity and diversity.
Immunoglobulins are distributed throughout bodily fluids including blood, lymph, saliva, tears, breast milk, and mucosal secretions, where they provide specific immune protection. Their ability to recognize and bind to specific molecular patterns on antigens (epitopes) enables them to neutralize pathogens, activate complement systems, enhance phagocytosis, and prevent microbial adhesion to mucosal surfaces. The immune system’s ability to remember previously encountered antigens and mount a rapid, specific response upon re-exposure (immunological memory) is largely mediated by immunoglobulins produced by memory B cells.
2 Structure of Immunoglobulins
2.1 Basic Architecture
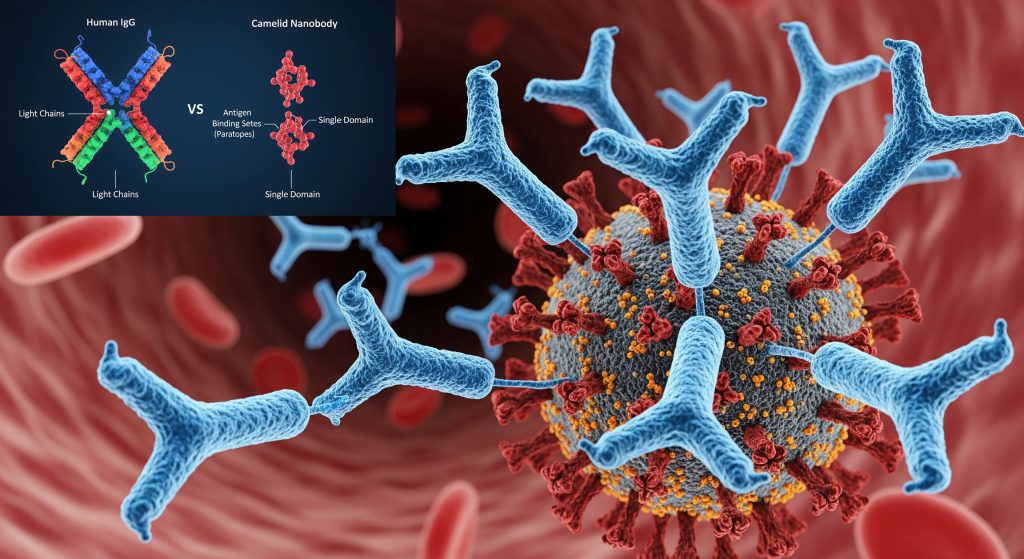
All immunoglobulins share a common Y-shaped structure composed of four polypeptide chains: two identical heavy chains (approximately 50,000 Da each) and two identical light chains (approximately 25,000 Da each). These chains are held together by a combination of non-covalent interactions and covalent interchain disulfide bonds, forming a bilaterally symmetric structure with a molecular weight of approximately 150,000 Da for monomeric immunoglobulins.
Each chain consists of constant (C) and variable (V) regions. The variable regions of heavy and light chains (VH and VL) comprise the antigen-binding sites (paratopes) that recognize specific epitopes on antigens. The tremendous diversity of these variable regions allows the immune system to recognize virtually any antigenic structure. The constant region determines the immunoglobulin’s class and functional properties, including its ability to activate complement, bind to cellular receptors, and traverse placental barriers.
2.2 Functional Fragments and Regions
Proteolytic cleavage of immunoglobulins reveals functionally distinct fragments. The Fab (fragment, antigen-binding) region contains the antigen-binding sites and consists of the entire light chain and the variable and first constant domains of the heavy chain. The Fc (fragment, crystallizable) region mediates biological functions such as complement activation and binding to cellular receptors. The hinge region, located between the first and second constant domains of the heavy chains in IgG, IgA, and IgD, provides flexibility allowing the two antigen-binding sites to operate independently.
2.3 Genetic Basis of Diversity
Immunoglobulin diversity is generated through somatic recombination of gene segments. Heavy-chain genes are assembled from four gene segments: variable (V), diversity (D), joining (J), and constant (C). Light-chain genes (κ and λ) are assembled from three gene segments: V, J, and C. This genetic rearrangement, combined with junctional diversity and somatic hypermutation, creates an enormous repertoire of antibodies capable of recognizing countless antigens.
3 Types of Immunoglobulins and Their Functions
The five primary classes of immunoglobulins—IgG, IgM, IgA, IgD, and IgE—are distinguished by their type of heavy chain (gamma, mu, alpha, delta, and epsilon, respectively) and perform distinct functions in immune defence.
Table: Immunoglobulin Classes and Characteristics
| Type | Molecular Weight | Serum Concentration | Percent Total Ig | Primary Functions |
| IgG | 150,000 Da | 10-16 mg/mL | 75% | Secondary response; crosses placenta; opsonization; neutralization |
| IgM | 900,000 Da | 0.5-2 mg/mL | 10% | Primary response; complement activation; natural antibody |
| IgA | 320,000 Da (secretory) | 1-4 mg/mL | 15% | Mucosal protection; neutralization in secretions |
| IgD | 180,000 Da | 0-0.4 mg/mL | 0.2% | B cell receptor; function not fully understood |
| IgE | 200,000 Da | 10-400 ng/mL | 0.002% | Parasite defence; allergic reactions |
3.1 IgG
IgG is the most abundant immunoglobulin in serum (75-80% of total immunoglobulins) and has the longest half-life (approximately 23 days). It is the principal antibody in secondary immune responses and provides long-term immunity against previously encountered pathogens. IgG is the only immunoglobulin that can cross the placental barrier, providing passive immunity to the developing foetus. It also activates the classical complement pathway, enhances opsonization, and neutralizes toxins and viruses. The four IgG subclasses (IgG1-IgG4) have slightly different properties and functions.
3.2 IgM
IgM is the first antibody produced during primary immune responses and exists primarily as a pentamer with ten antigen-binding sites. This multivalent structure makes IgM particularly effective at agglutinating antigens and activating the complement system. Although present in lower concentrations than IgG, IgM is a potent activator of the classical complement pathway and serves as a B cell receptor in its monomeric form.
3.3 IgA
IgA exists in both monomeric (serum) and dimeric (secretory) forms and is the predominant immunoglobulin in mucosal secretions including saliva, tears, breast milk, and respiratory, gastrointestinal, and genitourinary tract secretions. Secretory IgA (sIgA) provides crucial protection at mucosal surfaces by preventing pathogen adhesion, neutralizing viruses and toxins, and agglutinating microorganisms. It is resistant to enzymatic degradation due to its association with a secretory component.
3.4 IgD and IgE
IgD is present in minute quantities in serum and primarily functions as a B cell receptor involved in lymphocyte activation and differentiation. IgE has the lowest serum concentration but plays critical roles in defence against parasites and mediation of allergic reactions 313. IgE binds to high-affinity receptors on mast cells and basophils, triggering degranulation and release of inflammatory mediators upon antigen exposure.
4 How Immunoglobulins Work in the Human Body
Immunoglobulins provide protection through several mechanisms that can be categorized into three primary functions: neutralization, opsonization, and complement activation.
4.1 Neutralization
Immunoglobulins neutralize pathogens and their toxins by binding to functional sites, preventing attachment to host cells and entry into cells. For example, IgG and IgA antibodies can neutralize viruses by binding to viral surface proteins required for cellular attachment or fusion, thereby blocking infection. Similarly, antibodies can bind to bacterial toxins, preventing their interaction with host cell receptors and subsequent cellular damage.
4.2 Opsonization
Immunoglobulins enhance phagocytosis through opsonization—the process of coating pathogens with antibodies to mark them for destruction. Phagocytic cells (macrophages, neutrophils) express Fc receptors that recognize the constant regions of antibodies bound to antigens. This recognition leads to engulfment and digestion of the opsonized pathogens, efficiently clearing infections.
4.3 Complement Activation
Antibody-antigen complexes can activate the complement system through the classical pathway. IgM and IgG antibodies are particularly effective complement activators. Complement activation results in: (1) formation of membrane attack complexes that lyse pathogens; (2) generation of opsonins that enhance phagocytosis; (3) production of chemoattractant that recruit immune cells to sites of infection; and (4) promotion of inflammatory responses.
5 Pros and Cons of Immunoglobulins
5.1 Benefits of Immunoglobulins
- Specific immunity: Provide highly specific protection against a vast array of pathogens
- Immunological memory: Enable rapid and robust responses upon re-exposure to antigens
- Multiple protective mechanisms: Neutralize, opsonize, and activate complement
- Mucosal protection: IgA prevents pathogen entry at mucosal surfaces
- Passive immunity: Transfer of antibodies provides immediate protection (e.g., placental transfer, breast milk)
5.2 Potential Limitations and Considerations
- Immunodeficiency disorders: Deficiencies in immunoglobulins lead to increased susceptibility to infections
- Allergic reactions: IgE-mediated hypersensitivity can cause ranging from mild discomfort to life-threatening anaphylaxis
- Autoimmunity: Autoantibodies can attack self-tissues, causing autoimmune diseases
- Adverse effects of therapy: Immunoglobulin therapy can cause headaches, fever, chills, and more serious complications like acute kidney injury or thrombosis
6 Immunoglobulins in Camel Milk
6.1 Concentration and Unique Structure
Camel milk contains significant concentrations of immunoglobulins, with studies reporting mean IgG concentrations of 0.718 ± 0.330 mg/mL in Kazakh camel milk. Like lactoferrin, immunoglobulin content in camel milk shows seasonal variation, with the highest values observed in winter.
The most remarkable feature of camel immunoglobulins is their unique structure. Unlike immunoglobulins from other species, camel immunoglobulins contain only two heavy chains, with light chains being absent. This “heavy-chain-only” structure provides several advantages, including smaller size, enhanced stability, and improved penetration into tissues and cellular compartments.
6.2 Colostrum Content
Camel colostrum contains exceptionally high concentrations of immunoglobulins that provide crucial passive immunity to newborns. Research has shown that IgG concentration in camel colostrum decreases dramatically from 132 mg/mL to 4.75 mg/mL throughout the first 7 days postpartum, with a significant drop occurring immediately after parturition. This high initial concentration ensures adequate immune protection for the vulnerable newborn.
6.3 Functional Advantages
The unique structure of camel immunoglobulins confers several functional advantages:
- Enhanced stability: The absence of light chains contributes to greater structural stability and resistance to degradation
- Better tissue penetration: The smaller size allows for improved penetration into tissues and cellular compartments
- Novel antigen recognition: The single-domain structure enables recognition of unique epitopes that may be inaccessible to conventional antibodies
- Reduced immunogenicity: The simplified structure may reduce immunogenicity when used as therapeutic agents
7 Benefits of Camel Milk Immunoglobulins
7.1 Enhanced Immune Defence
Camel milk immunoglobulins provide comprehensive protection against diverse pathogens including bacteria, viruses, and fungi. Their unique structure allows them to target conserved epitopes on pathogens, potentially offering broader protection against variant strains. The stability of camel immunoglobulins in the gastrointestinal tract enhances their effectiveness against enteric pathogens.
7.2 Autoimmune and Allergy Support
Camel immunoglobulins may help modulate immune responses in autoimmune conditions and allergies due to their unique structure and specificity. Some studies suggest that camel milk consumption can benefit individuals with autoimmune conditions, potentially through regulatory effects on the immune system. The anti-inflammatory properties of camel milk may also contribute to these effects.
7.3 Therapeutic Applications
Camel heavy-chain antibodies are currently being investigated for immune therapy in patients with various disorders including cancer, multiple sclerosis, and Alzheimer’s disease. Their small size, stability, and unique antigen-binding properties make them particularly valuable for diagnostic and therapeutic applications. Camelid-derived nanobodies (single-domain antibodies) have shown promise in targeting difficult-to-reach epitopes, such as enzyme active sites and viral clefts.
7.4 Better Digestibility
The absence of light chains and different structure may make camel immunoglobulins more resistant to digestion, potentially enhancing their efficacy in the gastrointestinal tract. This improved stability allows them to maintain their functional integrity throughout the digestive process, providing enhanced protection against gastrointestinal pathogens.
8 Applications in Health and Medicine
Immunoglobulins have well-established clinical applications:
- Immunodeficiency diseases: Replacement therapy for primary immunodeficiencies
- Autoimmune disorders: High-dose IVIG modulates immune responses in autoimmune conditions
- Infection prevention: Prophylaxis against infections in immunocompromised patients
- Antivenoms and antitoxins: Provide passive immunity against toxins and venoms
- Transplantation: Help prevent rejection in transplant recipients
- Neurological disorders: IVIG used in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy
- Cancer therapy: Monoclonal antibodies target specific tumour antigens
9 Potential Downsides and Considerations
9.1 Immune Overreaction
In rare cases, particularly in individuals with IgA deficiency, immunoglobulin preparations can trigger severe allergic reactions or anaphylaxis. Additionally, there is theoretical concern that immune overstimulation could potentially exacerbate autoimmune conditions in susceptible individuals, though evidence for this is limited.
9.2 Limited Availability of Camel Milk Products
Despite their potential benefits, camel milk immunoglobulins face several limitations:
- Limited availability: Commercial production of camel milk is limited outside traditional camel-rearing regions, making it less accessible and more expensive than other milk types
- Taste differences: Camel milk has a distinct, slightly salty taste that may be less palatable to those accustomed to bovine milk
- Research gaps: While promising, many studies on camel milk’s health benefits are preliminary, small-scale, or based on animal models, requiring more robust clinical trials
- Safety concerns: Traditional consumption often involves raw milk, which carries risks of foodborne illness
9.3 Considerations for Therapeutic Use
When considering immunoglobulin-based therapies, several factors should be considered:
- Route of administration: Oral administration may limit effectiveness due to digestive degradation, though camel immunoglobulins show better resistance
- Dosing regimens: Optimal dosing for various conditions requires further research, particularly for camel milk-derived products
- Standardization: Products may vary in immunoglobulin content and specificity, requiring standardization for consistent effects
- Cost considerations: Immunoglobulin therapies, particularly novel formulations, can be expensive
10 Conclusion
Immunoglobulins represent one of the most sophisticated components of the adaptive immune system, providing specific protection against a vast array of pathogens through multiple mechanisms. The discovery of heavy-chain-only antibodies in camel milk has expanded our understanding of antibody diversity and opened new possibilities for therapeutic applications.
Camel milk immunoglobulins, with their unique structure and enhanced stability, offer distinct advantages over conventional antibodies from other species. Their presence in camel milk contributes significantly to the recognized health benefits of camel milk consumption, particularly in regions where camel milk is traditionally used for its medicinal properties.
Ongoing research continues to reveal new potential applications for both conventional and camel-derived immunoglobulins in various fields of medicine. As our understanding of these remarkable molecules expands, so too will our ability to harness their potential for improving human health.
References
- Rasheed Z. Medicinal values of bioactive constituents of camel milk. PMC. 2017 Nov-Dec;11(5):1–2. 1
- Immunoglobulins – Structure and Function. Microbiology and Immunology Mobile. 3
- Immunoglobulins: Types and Functions. Lecturio. 13
- Konuspayeva G, et al. Lactoferrin and immunoglobulin contents in camel’s milk from Kazakhstan. J Dairy Sci. 2007 Jan;90(1):38-46. 12
- Azwai SM, et al. Immunoglobulins of camel (Camelus dromedarius) colostrum. J Dairy Sci. 1996;79:129-134. 7